The Rankine cycle

Much of what you see coming out of a coal plant is steam. Why would they throw away energy like that?
Usefulness of two phase systems
The Carnot cycle has us adding / extracting heat at a constant temperature.
An isothermal process (constant temperature) is reversible: If $T$ is constant then the change in entropy when you add heat to a system: $$\Delta s = \int_1^2 \frac{\delta q}{T}=\frac 1T \Delta q,$$ is the same as the change in entropy when you take heat out of the system: $$\Delta s = \int_2^1 \frac{\delta q}{T}=-\frac 1T \Delta q.$$
An ideal Carnot cycle is put together from reversible processes, and that's why it has the highest possible efficiency.
But practically speaking...how do you keep the temperature of a finite system constant while adding heat? Doesn't every system increase its temperature with the addition of heat??
Not a two-phase system. You can put heat in/out without changing its temperature.
The heat needed to change an amount of a substance from one phase to another at constant pressure/Temperature is the latent heat $l$ of vaporization: $$ Q = m l.$$
For water at room temperature, $l=538$ kCal / kg.
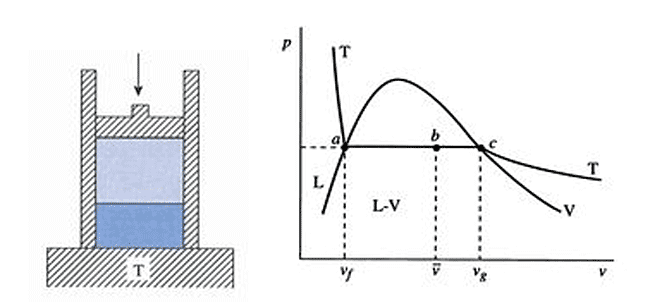
A two-phase system makes it easy to heat a system isothermally.
Let's put a Carnot cycle (isothermal, adiabatic, isothermal, adiabatic) in under the "dome" defined by the liquid-vapor coexistence region:
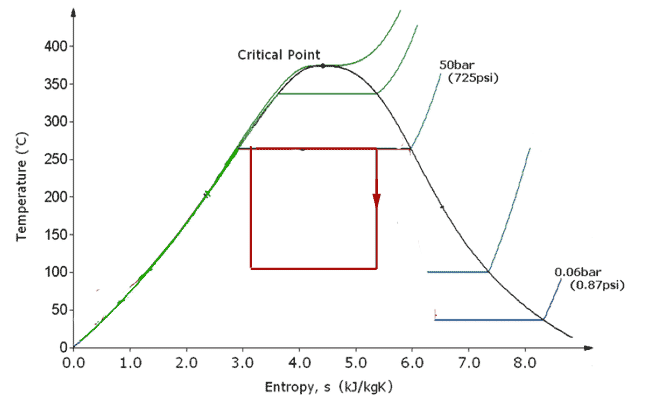
Unfortunately, there are some downsides to this as a practical device:
- If you compress a gas-liquid mixture too fast, $T_g$ will rise much quicker than $T_l$, there will be temperature gradients, and the system is no longer reversible. (A reversible cycle is quasistatic: *practically* always at/near equilibrium.)
- But if you compress too slowly, the available power (energy / time) drops.
- A higher efficiency could be attained with a higher $T_{max}$, but you're limited by the critical temperature.
- Condensation in the adiabatic part of the cycle (in a turbine) can cause more rapid wear and reduced efficiency. (Imagine a rapidly spinning propeller, and then you pass air through it with small droplets of water.)
The Rankine cycle...and beyond
The Rankine cycle--used for ~80% of electric generation worldwide, burning coal / natural gas or nuclear power...
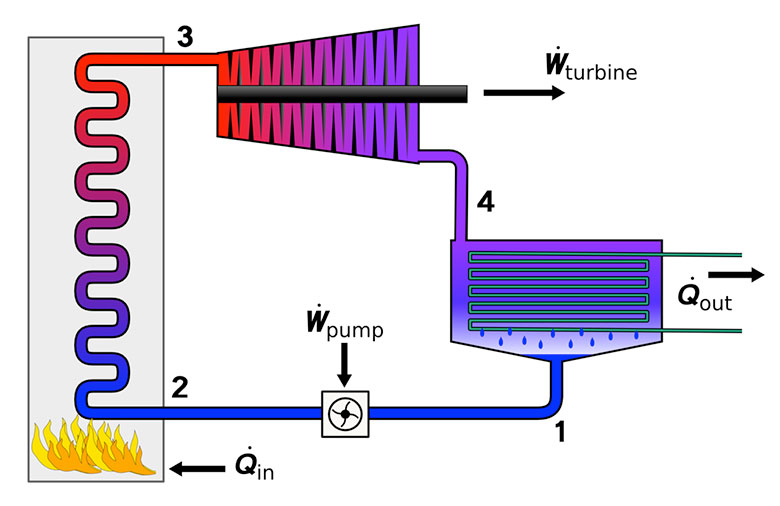
...is a compromise between high efficiency and high power:
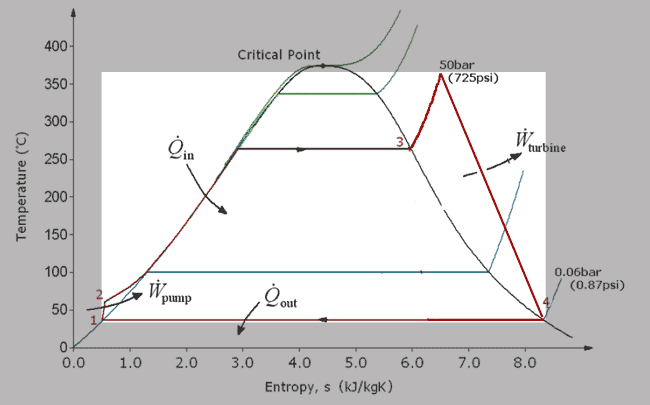
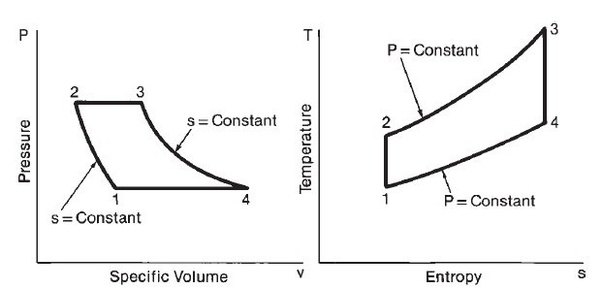
[Recall--A Carnot cycle is a rectangle on a $T-S$ diagram, and the area is the work done.]
- Compression takes place outside of the coexistence region, where the water is liquid only.
- Expansion takes place with 'dry' vapor.
- *Lots* of heat enters the system in the constant temperature region.
Actual Rankine cycle engines have efficiencies ~40%, compared to a Carnot efficiency of ~60%. (Gasoline (Otto cycle) engines reach only ~15-25%).
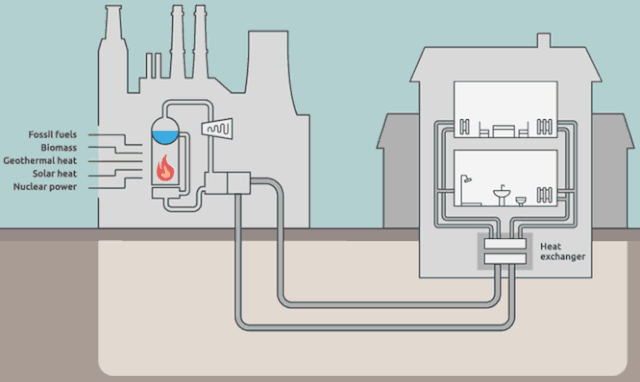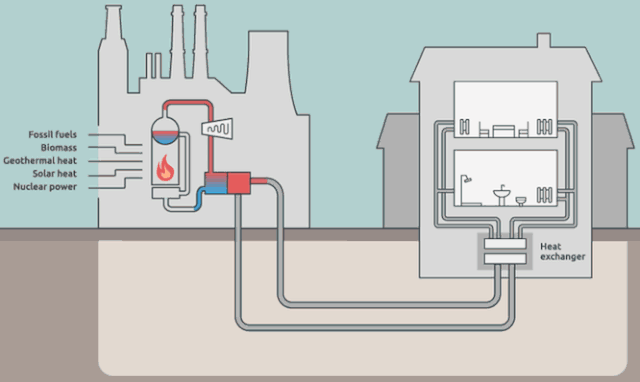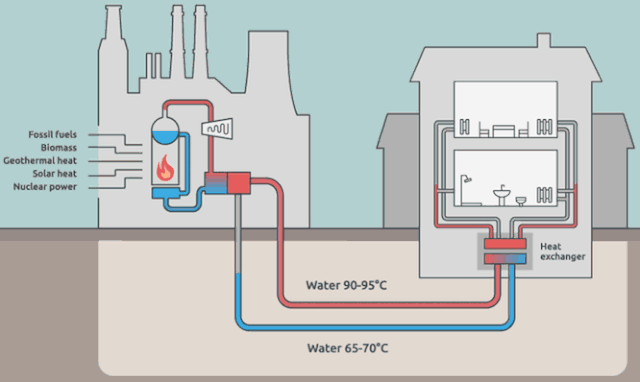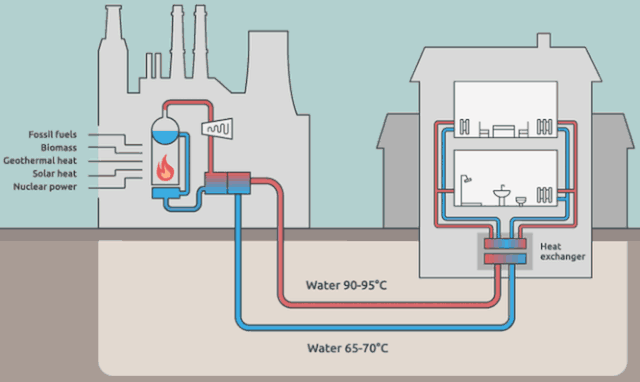
Why cool the steam?

Step 4 is where the steam in the system (water is the "working fluid" of the Rankine cycle) is being cooled back to liquid water.
The steam in the picture below is actually coming from the water in the external cooling loop.

Is it really necessary to cool down the working-system steam? Aren't we just flushing away (heat) energy? What would happen if we *didn't* cool the steam? Wouldn't we have a greater efficiency?
The Carnot cycle efficiency is calculated as the ratio of $W_\text{out}/Q_\text{in}$: It's the fraction of your input energy (heat) which is converted to mechanical energy (work).
But your *energy* efficiency can be higher than the Carnot efficiency, if you have a use for $Q_\text{out}$. This is the idea behind District Heating.
Laura Toffetti, DensityDesign Research Lab
The Brayton cycle
...describes a gas turbine or jet engine. The system never gets into the coexistence regime. It operates at high temperatures. See Spakovsky on the Brayton cycle.

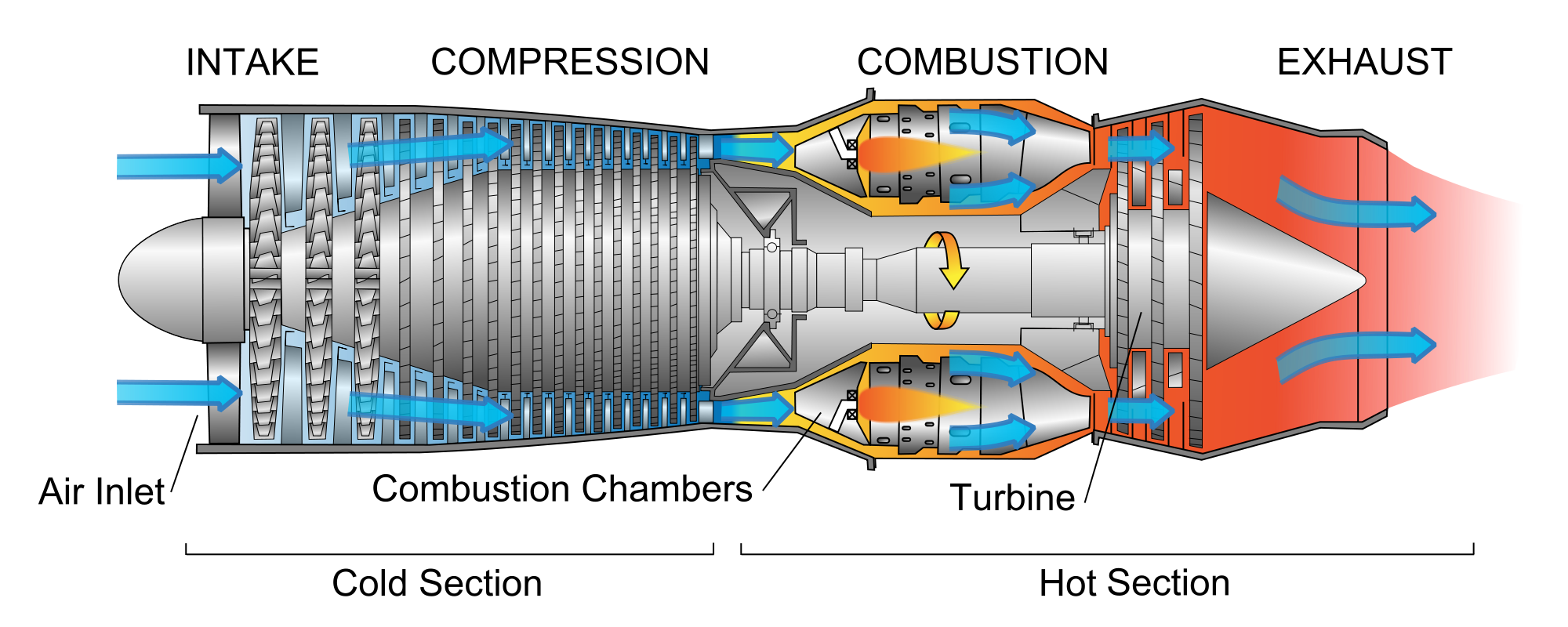
The jet engine is not the system. The "system" is a chunk of air, initially at STP, that starts out outside (in front of) the jet engine, and then moves in to the engine.
It's not *really* a closed system, (the output air molecules are not re-directed back in to the input of the engine), so what's the justification in drawing a "cycle"?
- Provide a "story" of what happens to your chunk of air as it passes through the jet engine, with reference to the numbers on the diagram above. Among other things: Where is your little chunk of air during the isobaric cooling part of the diagram? One of the steps is a compression. What *kind* of compression is that part of the process? Where in the cycle is the combustion happening?
- Thinking now of the speed of the center of mass of your little chunk of air relative to the jet engine: When your chunk is in the highest-pressure part of the engine how fast is it moving compared to when it was outside?
- Spakovsky's question: Can we run this backwards to do refrigeration?
Two more questions to turn over in your mind...though you may not have immediate answers: Why are we (both here and in the Rankine cycle) always compressing the working fluid before heating? What would happen if we tried to run this cycle without the compression--or even more extreme--by decompressing the working fluid before heating?
- Chunk of air enters the front of a jet engine,
- It's compressed. What kind of process is this??
- Jet fuel added and ignited,
- Expands, pushing on turbine blades,
- Ejected from the engine, gives up its heat.
In response to Ebtihal's question... A Brayton cycle natural gas turbine has a typical efficiency of 35-45% on its own.
Combined cycle gas turbine (CCGT)
...Consists of a gas turbine operating a Brayton cycle at high temperature (the "topping" cycle), and a conventional steam unit (Rankine cycle) at lower temperatures (the "bottoming" cycle).
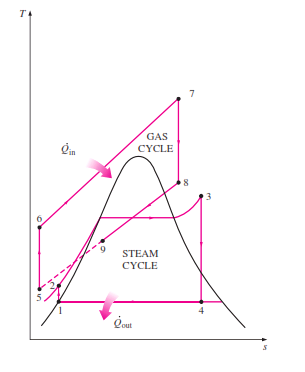
The exhaust heat
from the topping cycle (coming out at above 500 C)
is used as input heat for the bottoming cycle.
The resulting combined cycle has the high temperature of a gas turbine, and the low temperature of a Rankine cycle, resulting in higher Carnot efficiency Commercial units have reached 62%, and appear to be approaching 64% efficiencies.
Carbon can be captured, at an approximate cost of 10% of cycle efficiency, so we're down around 54-56% net efficiency with carbon capture.
Carbon Capture, and the Allam cycle

The Allam cycle (Science, 2017) uses supercritical $CO_2$ as a working fluid. Supercritical $CO_2$ has properties of a gas (filling a container) with the much higher densities of a liquid. Compared to a traditional Rankine cycle that uses air/steam vapor as the working fluid:
- Smaller volume of fluid needed to attain the same momentum transfer to turbines $\Rightarrow$ turbines can be made much smaller/cheaper (and mass produced),
- Smaller volume of liquid contains much greater density of thermal energy $\Rightarrow$ smaller heat exchangers.
- $sCO_2$ is not nearly as corrosive as steam.
- Lower cost (capital cost and energy cost) pumps to compress already high-density super critical liquid.
- Maximum efficiencies thought to be 50-54% - comparable to CCGT with CCS add-ons.
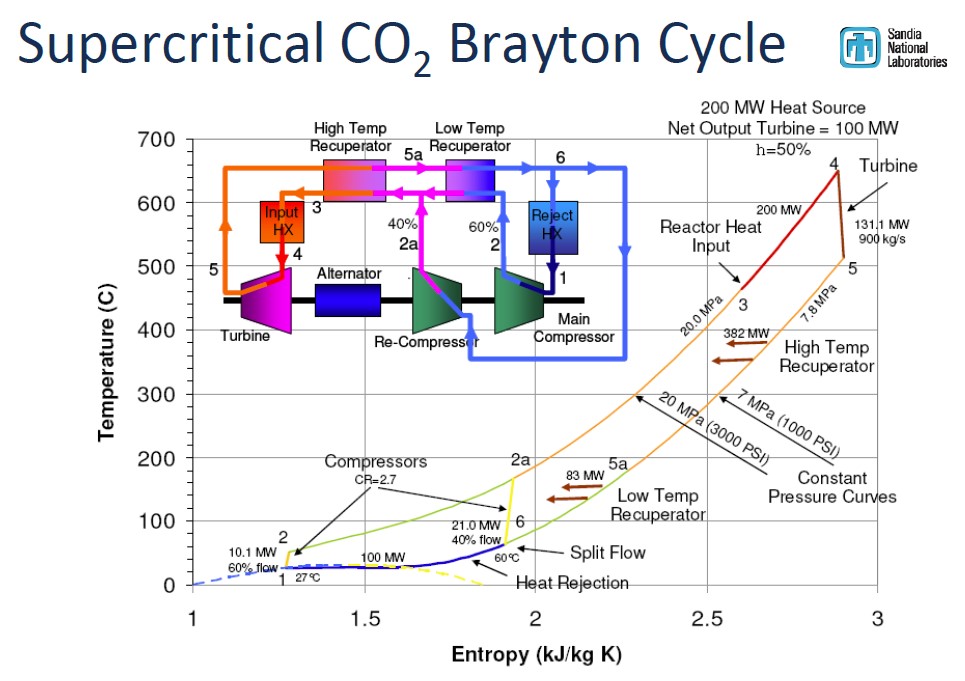
- Natural gas combustion with pure oxygen (no nitrogen) means working fluid stays 97+% $CO_2$. So, no need for extra hardware to separate the $CO_2$ from other flue gases. Pure carbon emissions come out in a pressurized stream.
- Only other emission is condensed water from combustion: The power plant produces purified water rather than requiring a water cooling source.
- The oxygen separation plant does offset some other savings.
- Apart from the high-pressure $CO_2$ stream, which is ready to be piped to carbon capture system, no atmospheric emissions!
- Like more traditional natural gas power plants, an Allam system can be turned on and off relatively quickly compared to a nuclear power plant (~a day) or even a traditional coal-fired plant. So it can complement intermittent renewable energy sources.
- Lower capital costs and lower land use requirements compared to CCGT systems.
Image credits
Earthtimes, Andrew Ainsworth, Ritu Raj Kumar?, Lee Hutchinson, BBC, Sandia national lab via BreakingEnergy.com