Defining thermodynamic SYSTEMS
Systems, thermodynamic states, variables, and more...
"System"
He drew a circle that shut me out
- Heretic,rebel, a thing to flout.
But love and I had the wit to win:
We drew a circle and took him In!
--Edwin Markham
 Imagine (or draw) any boundary in space.
Imagine (or draw) any boundary in space.- But your boundary must have an inside, which we'll call a thermodynamic system.
- Outside the boundary is the surroundings.
- Together, the system and the surroundings constitute a universe.
Like Harold, You have the power to define the boundary of a system in whatever way is easiest to deal with a particular problem!
[Harold and the Purple Crayon is a classic children's book written in 1955. Here's an animated version.]
Closed systems
 Think of the system boundary as coincident with the latex of one of these balloons.
Think of the system boundary as coincident with the latex of one of these balloons.
If the matter, or other stuff inside the system can *not* be exchanged with the surroundings, the system is closed.
"Stuff" might be atoms or radiation (photons).
If the "stuff" is atomic, it might be in any phase (solid / liquid / gas / plasma).
Note: In some textbooks you'll hear talk of a "change of state" when a material goes from from liquid to gas. In this course we'll call that a phase change. The word "state" has a different, and very specific meaning in thermodynamics.
The "stuff" might be atoms that re-arrange their chemical bonds.
... vs Open systems
 Think of the system as consisting of the space inside the cooler when the lid is on.
Think of the system as consisting of the space inside the cooler when the lid is on.
If matter *can* be exchanged with the surroundings--that is, if matter can cross the system boundary--then the system is open.
Adiabatic systems
 Think of the system as the space inside the thermos (not in the insulation), when the lid is on.
Think of the system as the space inside the thermos (not in the insulation), when the lid is on.
A boundary is adiabatic if heat cannot cross the boundary of the system.
An isolated system is both:
- adiabatic (no heat exchange), and
- closed (matter cannot cross the system boundary).
...vs Diathermal
 Think of the system as the inside of the ice pack when the lid is on.
Think of the system as the inside of the ice pack when the lid is on.
Heat (or cold) *can* cross a Diathermal system boundary.
During the rapid compression of our pop can... Consider the can to be the system boundary: Was the system open or closed?
Consider the bullet of question 1-2 f...why was it an "adiabatic" system?
Fixed or changing volume
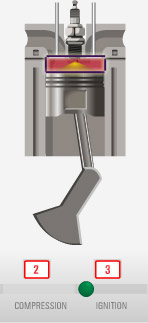 The
boundary may be moving or changing with time.
The
boundary may be moving or changing with time.
Think of our system as consisting of the space above the (moving) piston and below the inlet/outlet valves. The volume of this space is changing with time.
To do: Sketch approximately $V(t)$.
In general physics, work is given in terms of displacement and force: $$\delta W = \myv{F} \cdot \delta \myv x$$
To do: Under what circumstances can a system not possibly do any mechanical work on its surroundings (or vice versa).
Image credits
Frederick Wilson, Flickr users Jaxxon, Swamibu, Matus Kalisky, Windows to the Universe, Wikimedia user Zephyris