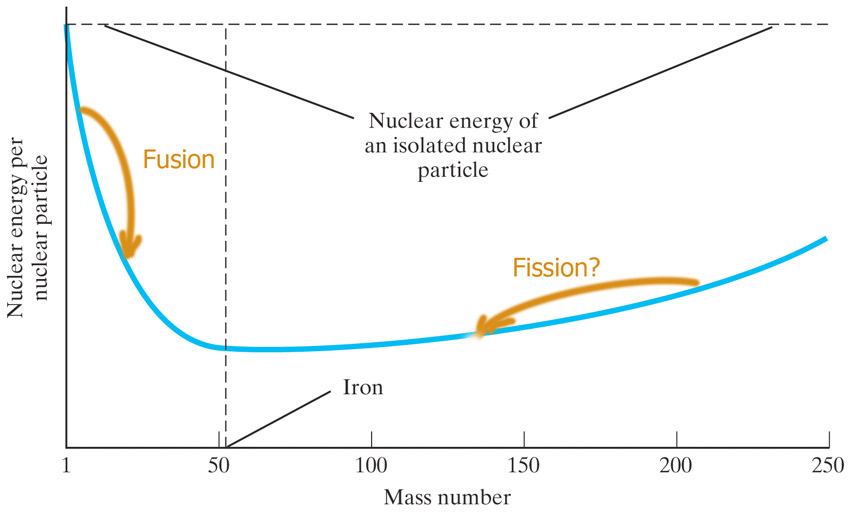
Fusion
We've been studying spontaneous nuclear decays and fission (nucleus splits).
Inside of stars and nuclear reactors the opposite process also happens to: fusion.
Separate vs together
Consider a neutron and a proton that interact through the strong force. Are they attracted to each other or repelled?
Is there any electric force between a neutron and a proton?
So, what would you expect: Does it take work to push them together or pull them apart?
(free neutron and proton) ${}_0^1n + {}_1^1p \leftrightarrow {}_1^2 H$ (bound together)
higher nuclear energy $\leftrightarrow$ lower nuclear energy
higher NuclE $\rightarrow$ lower NuclE + ThermalE + RadiantE
or: NuclE $\rightarrow$ ThermalE + RadiantE
In the sun
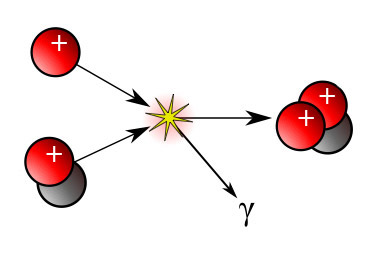 This
reaction is part of the proton-proton
chain reaction by which the sun creates alpha particles ($He$) from
protons:
This
reaction is part of the proton-proton
chain reaction by which the sun creates alpha particles ($He$) from
protons:
$${}_1^1H + {}_1^2H \rightarrow {}_2^3He + gamma $$
But unlike the the $n+p$ reaction, both nuclei are positively charged. So this can only happen with lots of input energy...
ThermalE${}_{i n}$ + NuclE $\rightarrow$ ElectricE + RadiantE + (gobs of) ThermalE${}_{o u t}$
This one also releases energy
Missing mass?
It's possible to weigh different isotopes to high precision. When you do this you find...(in units of $10^{-27}$kg):
| mass of ${}_1^1H=1.6727$ |
|
| $5.0164 $ |
The difference is $0.0098 \times 10^{-27}kg$.
Energy weighs something
 What's the most famous
equation associated with this man?
What's the most famous
equation associated with this man?
$E=mc^2$
$c=3 \times 10^8$m/s=speed of light.
$0.0098 \times 10^{-27}$kg $* (3 \times 10^8$m/s$)^2 = 8.82 \times 10^{-13}$J
...turns out to be the energy released when this reaction takes place.
Elements heavier than Helium?
The Big Bang should have produced nothing but H, He, and bits of Li.
Which side of the equation weighs more? (Has more energy) Use Wolfram Alpha to look up the "weight of He-4"...
${}_2^4He + {}_2^4He \stackrel{?}{\leftrightarrow} {}_4^8Be$
${}_2^4He + {}_4^8He \stackrel{?}{\leftrightarrow} {}_6^{12}C$
WolframAlpha gives answers in "u" = unified atomic mass units. $$1\ \text{u}=1.66053892\times 10^{-27}\ \text{kg}$$ This is 1/12 of the weight of 1 mole of carbon-12.
We found...
${}_2^4He + {}_4^8He \rightarrow {}_6^{12}C$
But:
${}_2^4He + {}_2^4He \leftarrow {}_4^8Be$
The nuclear energy curve
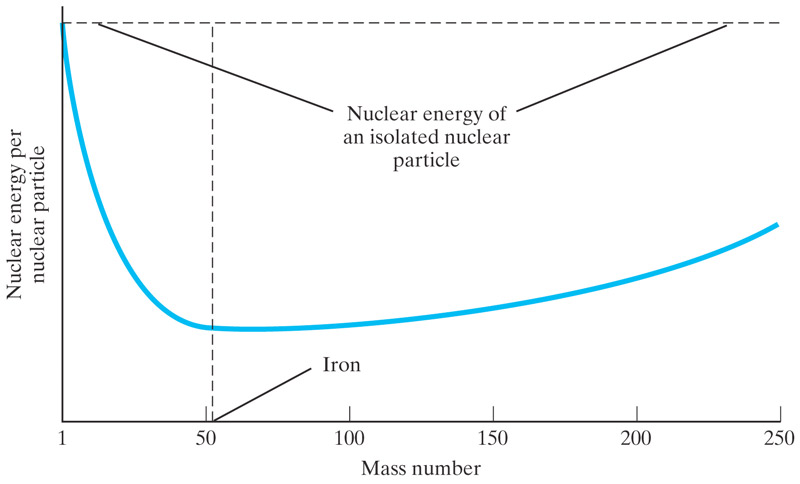
Elements beyond iron
There is less certainty about how the elements beyond iron formed.
 The most prominent view is that these are formed by the shock wave of supernova explosions, which also serves to distribute the elements to the rest of the universe.
The most prominent view is that these are formed by the shock wave of supernova explosions, which also serves to distribute the elements to the rest of the universe.
Splitting the atom
Suggested exercises
Chapter 16, Conceptual Exercises: 5, 7, 10, 17