

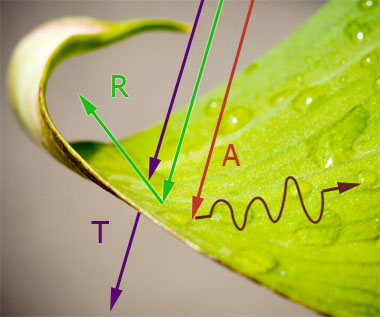
 Three kinds of things might happen when light strikes something, depending on the wavelength of the light, and the material it hits:
Three kinds of things might happen when light strikes something, depending on the wavelength of the light, and the material it hits:
Glass (and carbon dioxide) are like this:
How do we know?
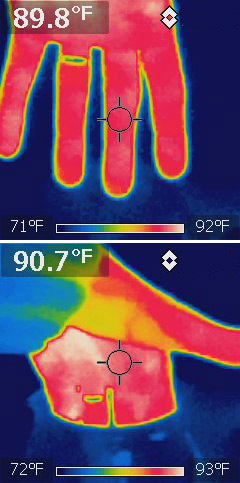
...that water does not transmit microwave light?
(Think of what happens in a microwave oven as microwave 'light' shining on water).
Here's a scrambled explanation of the 'greenhouse effect' in a parked car. Put these pieces in order...
In earth's atmosphere, substitute "carbon dioxide, water vapor, methane, and some other gases" for "windshield"!
If you were designing a system to sense nearby objects by detecting the E-M waves bouncing off of them, and you could only use a small range of wavelengths--what wavelength of light should you use??
There are animals that are sensitive to light in each of these regions:
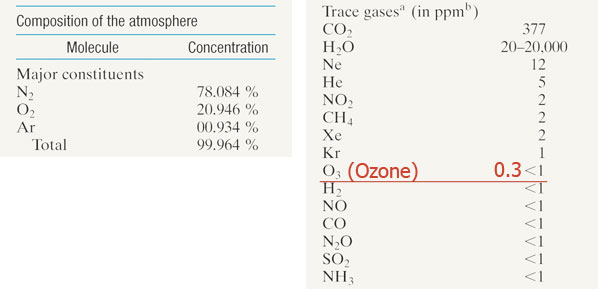
The SHIELD that protects us from ultra-violet (high energy) light from the sun...
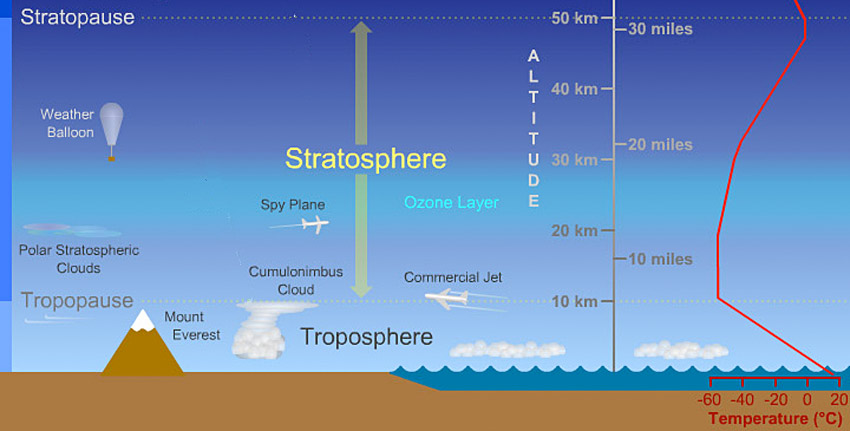
90% of Earth's air mass is within 10 km of the surface of Earth.
Around 1900 - Refrigerators use toxic gases as coolants: methyl chloride, ammonia, sulfur dioxide.
 1928 -
GM chemists synthesize a chlorofluorocarbon (CFC), brand-named
"Freon" used first in Frigidaire refrigerators: non-toxic, non-corrosive, chemically
inert. Breathe it, and nothing happens to you!
1928 -
GM chemists synthesize a chlorofluorocarbon (CFC), brand-named
"Freon" used first in Frigidaire refrigerators: non-toxic, non-corrosive, chemically
inert. Breathe it, and nothing happens to you!
1932 - Carrier uses Freon in its "Atmospheric Cabinet" - world's first self-contained home air-conditioner unit.
1945 - Freon used as the propellant in paints, hair sprays, health care products. Later, foam bubbling agent.
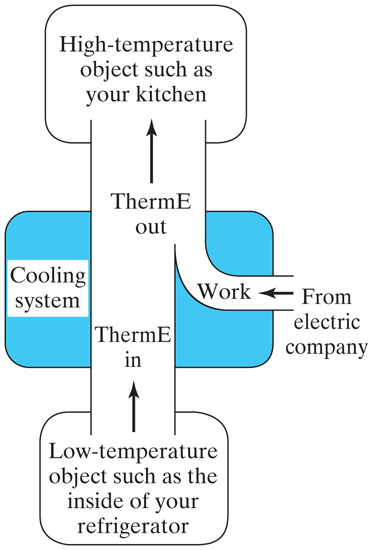 Refrigerators,
air conditioners, and heat pumps all use a "heat engine in reverse" to transfer
thermal energy out of something cold, and dump the thermal energy
into a warmer location.
Refrigerators,
air conditioners, and heat pumps all use a "heat engine in reverse" to transfer
thermal energy out of something cold, and dump the thermal energy
into a warmer location.
When a liquid evaporates, it absorbs energy from its surroundings.
Mechanical energy is used to re-compress the evaporated liquid on the 'hot' side.
 1974 -
Mario Molina and Sherwood Rowland suggested that CFCs could be broken apart
by high energy UV light, freeing
chlorine, which can then do this...
1974 -
Mario Molina and Sherwood Rowland suggested that CFCs could be broken apart
by high energy UV light, freeing
chlorine, which can then do this...
`Cl + O_3 \rightarrow ClO + O_2`
A second reaction frees the Cl to destroy anew...
`ClO + ClO + [UVl i g h t] \rightarrow Cl+Cl+O_2`
1978 - Consumer boycott leads to U.S. ban on CFCs in spray propellants.
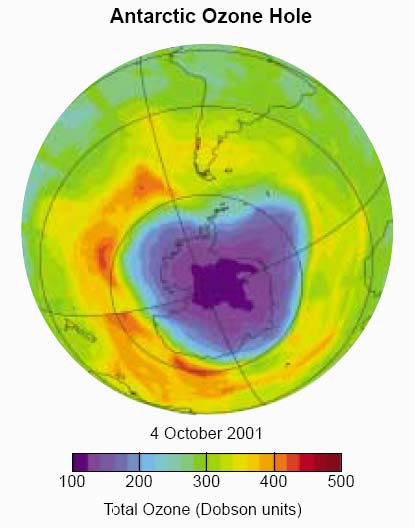
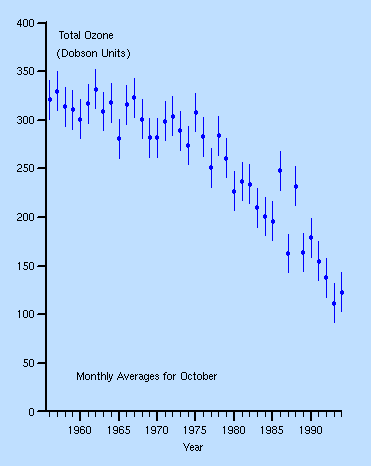 English researchers at the Halley
Bay research station in Antarctica had been measuring ozone since the late
1950s. They couldn't quite believe the drop after ~1975, checked and re-checked,
and published in 1985.
English researchers at the Halley
Bay research station in Antarctica had been measuring ozone since the late
1950s. They couldn't quite believe the drop after ~1975, checked and re-checked,
and published in 1985.
They also noticed a new seasonal effect: Each spring (October), the ozone would suddenly drop precipitously, and then recover a few months later.
 Hazards
of elevated UV radiation include
Hazards
of elevated UV radiation include
 1986 -
Susan Solomon organized an expedition to measure ClO.She proposes that stratospheric
ice breaks up enclosed CFCs, releasing Cl when heated by springtime sunlight.
1986 -
Susan Solomon organized an expedition to measure ClO.She proposes that stratospheric
ice breaks up enclosed CFCs, releasing Cl when heated by springtime sunlight.
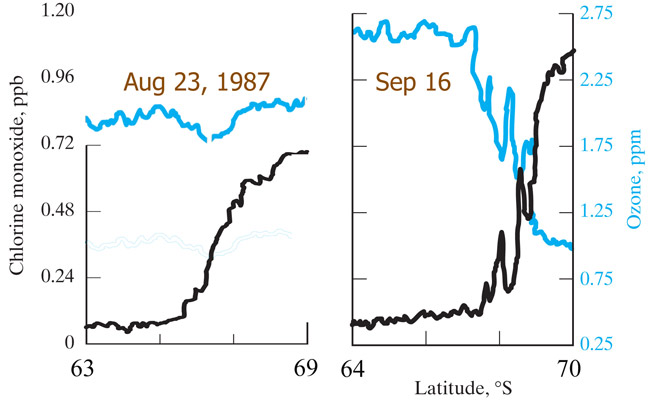
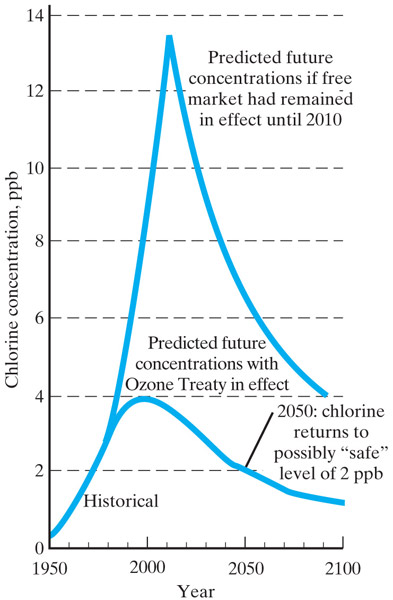 1987 -
24 nations signed the Montreal Protocol. As of Sept
2009, all 196 member states of the UN had signed on to the treaty to phase
out ozone-destroying chemicals.
1987 -
24 nations signed the Montreal Protocol. As of Sept
2009, all 196 member states of the UN had signed on to the treaty to phase
out ozone-destroying chemicals.
1995 - Nobel prize in Chemistry goes to Paul Crutzen, Mario Molina and Sherwood Rowland. The committee commends them for "contribut[ing] to our salvation from a global environmental problem that could have catastrophic consequences."
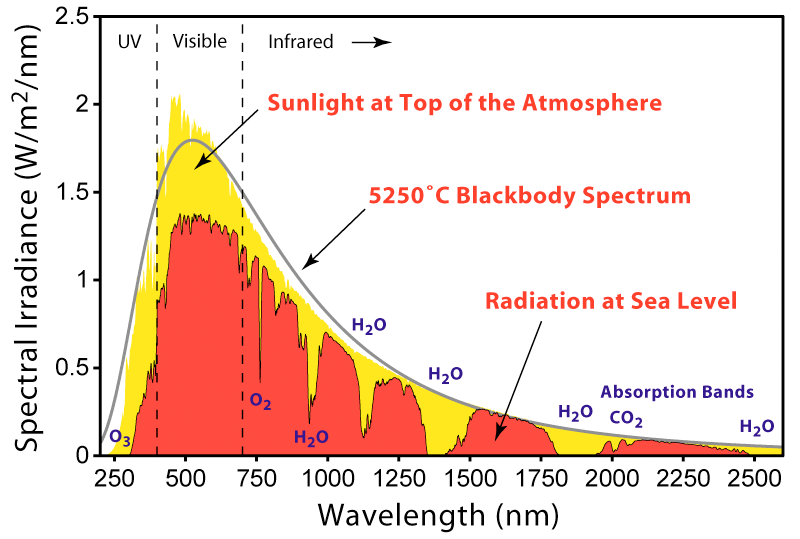
Solar spectrum - Globalwarmingart.com, Windows to the Universe, Jason Swaby, IPCC