 This
is no accident: Sound is a pressure disturbance travelling through air.
It seems plausible that any disturbance should move with the same underlying
speed as the atoms are moving.
This
is no accident: Sound is a pressure disturbance travelling through air.
It seems plausible that any disturbance should move with the same underlying
speed as the atoms are moving.We saw that Conservation of Energy only worked once we could precisely account for transformations of other energies into ThermalE.
What is thermal energy?
At room temperature, a typical speed is $v$ ~ 700 mi / h - close to the speed of sound.
 This
is no accident: Sound is a pressure disturbance travelling through air.
It seems plausible that any disturbance should move with the same underlying
speed as the atoms are moving.
This
is no accident: Sound is a pressure disturbance travelling through air.
It seems plausible that any disturbance should move with the same underlying
speed as the atoms are moving.
What is this in miles / sec? ...and in sec / mile? This time, see WolframAlpha
.Light travels at 3 X 10^8 m /sec = 186,000 miles/sec: much faster than sound.
 Use this to figure out... How far away is this
airplane?
Use this to figure out... How far away is this
airplane?
By the way... According to this USA Today article, civil aircraft are hit on average once per aircraft per year. But lightning hasn't caused any airplane to crash since 1981.
 The
metric system uses the Celsius degree to measure temperature.
The
metric system uses the Celsius degree to measure temperature.
0 C = water freezes = 32 F
100 C = water boils = 212 F
37 C = normal human body temperature = 98.6 F
40 C = bad fever = 104 F
1 C = 9/5 ${}^o$F~2${}^o$F
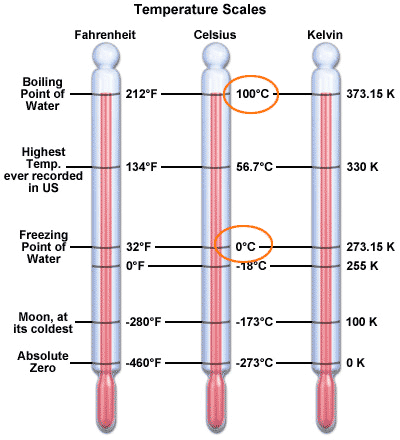
But what is temperature?
Thermal energy is the disorganized kinetic energy of atoms.
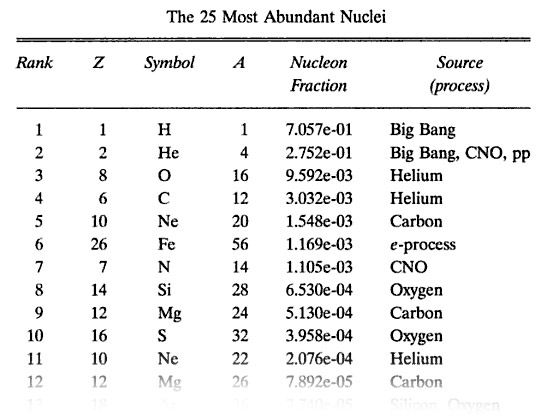
Hydrogen is the most abundant atom in the universe. 70.6% of the atoms in our solar system are hydrogen:
 Jupiter's atmosphere: 90% is $H_2$.
Jupiter's atmosphere: 90% is $H_2$.
 Saturn: 75% $H_2$.
Saturn: 75% $H_2$.
Earth: 0.000055 % $H_2$.
??
Actually, Temperature is not proportional to the average speed of molecules, it is proportional to the average kinetic energy:
$$T\propto {\rm KineticE} = \frac{1}{2}mv^2.$$
 If
two atoms have the same kinetic energy (that is, the same temperature),
but different masses, which one is moving faster?
If
two atoms have the same kinetic energy (that is, the same temperature),
but different masses, which one is moving faster?
Hydrogen gas ($H_2$) has an atomic weight of 2 grams / mole.
Nitrogen gas ($N_2$) has an atomic weight of 28 grams / mole. So, one nitrogen molecule has a mass of 14 times the mass of a hydrogen molecule.
If we have any hydrogen in the atmosphere along with the 78% of our atmosphere that's nitrogen, how much faster is the hydrogen moving?
$$\frac{1}{2}m_{H_2} v_{H_2}^2=\frac{1}{2}14*m_{H_2}(760 {\rm mph})^2.$$
With a little algebra...(and help from Google) $$v_{H_2} = \sqrt{14}*760 {\rm mph}= 2,844 mph = 1.3 km /sec.$$
This is about 10% of the escape velocity -- 11 km/sec -- from earth.
So occasionally, a hydrogen molecule will get going fast enough to reach escape velocity.
Remember this: at the same temperature, light atoms move faster than heavy atoms
We think the planets all formed from the same kind of dust--with roughly the same chemical composition, and yet their atmospheres are quite different--as we've seen with hydrogen. Let's see if we can account for another gas that's present on some planets and not on others:
So as you cool things down, the atoms are moving slower and slower. At a certain temperature they would stop completely:
-273 C = absolute zero
This is the basis of the Kelvin temperature scale: the degrees are the same size, but the zero point is absolute zero.
Room temperature = 20 C = 273 + 20 = 293 K.
There is a very simple formula relating kinetic energy per molecule to temperature (in Kelvin) it is...
$$K.E. = 2 \times 10^{-23} T_K.$$
So, the formula for thermal energy is related to the $N$--number of molecules-- and the temperature (in degrees Kelvin), like this
$$ThermalE = 2 \times 10^{-23} NT_K.$$
Which has more thermal energy? A mug of hot chocolate or a mug of ice water?
A mug of hot coffee or Lake Michigan at room temperature?
The Lake, even though it's at a lower temperature, still has lots more microscopic kinetic energy then a mere mug of hot coffee because the number of molecules $N$ in the lake is so much larger.
In general... do things get bigger or smaller when you heat them?
 The most familiar thermometers depend on a change in volume of alcohol (colored red). Years ago mercury was used. The glass enclosing the alcohol is also expanding. But not nearly as much as the alcohol.
The most familiar thermometers depend on a change in volume of alcohol (colored red). Years ago mercury was used. The glass enclosing the alcohol is also expanding. But not nearly as much as the alcohol.

 Thermal expansion/contraction is the reason we put neat cracks into sidewalks, and complicated cracks ("expansions joints") into bridges.
Thermal expansion/contraction is the reason we put neat cracks into sidewalks, and complicated cracks ("expansions joints") into bridges.
If you have a lid that's too tight Should you run hot water on the jar or the lid??
 Loosening lids by running under hot water uses thermal expansion.
Loosening lids by running under hot water uses thermal expansion.
Close fitting ball and ring... What happens if you
Typical thermal expansions are around... $$\frac{\Delta V}{V} =~ 10^{-4} / {}^o C$$ This means, if you increase the temperature by 1 degree C, the volume will increase fractionally by 1/10,000
Current predictions for average warming due to excess $CO_2$ are 2-4 C by 2100.
Even if all the ice (and glaciers) did *not* melt, the ocean could still rise due to thermal expansion. How much? Taking the higher value of $\Delta T = 4$ C,
Water has an expansion coefficient at room temperature of $ \frac{\Delta V}{V} = 2.0 \times 10^{-4} / {}^o C$ and the oceans have an average depth of 12,000 feet.
If the ocean is like a glass which is filled to a depth $h$,
$$\begin{align}\Delta h & = h \cdot 2.0 \times 10^{-4} \cdot \Delta T \\ &=(12,000 ft)(2.0 \times 10^{-4})(4 C) = 9.6 {\rm \ feet}\end{align}.$$ This is enough to be worrisome.
The IPCC has done a more detailed calculation. Luckily:
Their prediction is a maximum rise of 1.9 feet. If you add in the effect of polar ice melting, the maximum sea level is more like 2.5 feet.
You can explore the effect of a 3 ft rise with this Google Maps sea level simulator.